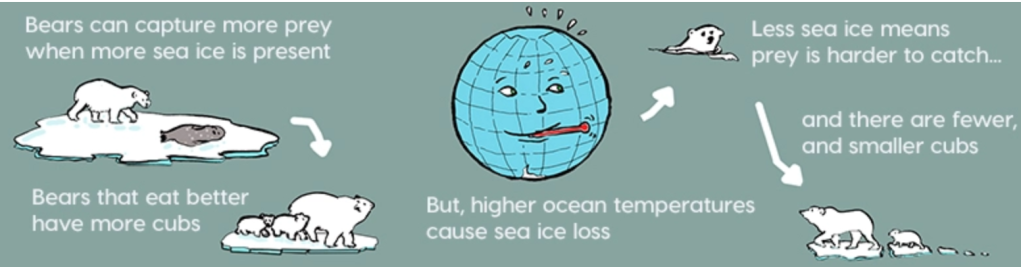
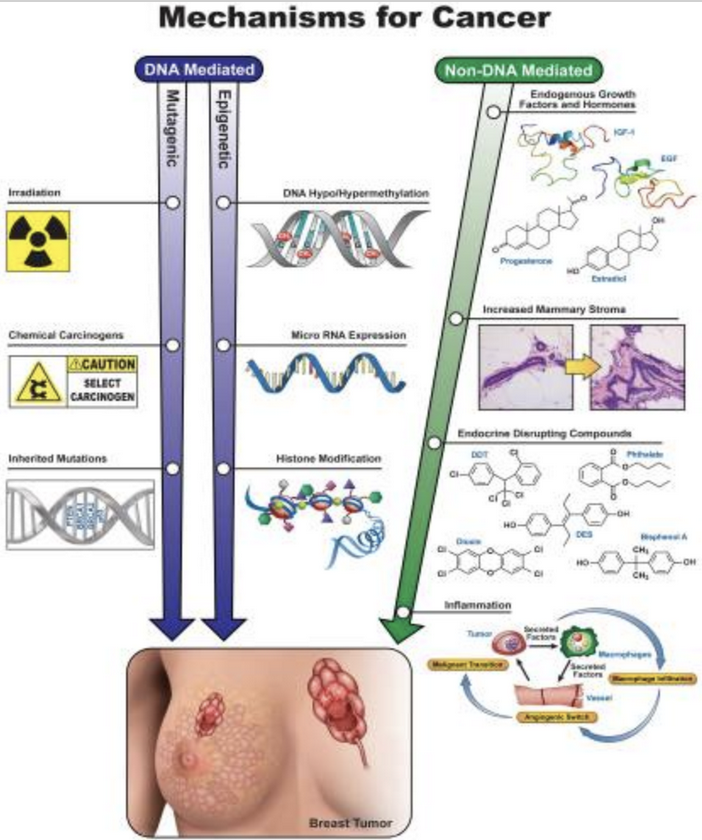
Need a bit of inspiration to face our uncertain future? Look no further than the community organizers of Cancer Alley. Since the 1970’s these phenomenal people have stood up to multi-billion-dollar petrochemical companies. These companies, empowered by municipal planning boards and powerful politicians, release tons of carcinogenic and endocrine disrupting chemicals in the air and water, thereby sacrificing the health and lives of the good people who happen to live in nearby communities. In this post, based on research done by Lehigh student, Reese Keveanos, you’ll be introduced to activists and community organizers like Sharon Lavigne, a grandma and former special ed teacher who was awarded the prestigious Goldman Environmental Prize. She founded the Rise St. James organization, and so far, she and her allies have been able to delay construction of the several billion-dollar Formosa plastics complex, thereby preventing it from further polluting her Louisiana neighborhood.
Be sure to read through this post to find concrete ways you can help!
One Oppressive Economy Begets Another
Cancer Alley, also known as the Mississippi River Chemical Corridor, is an 85 mile strip of land that snakes from New Orleans to Baton Rouge. After the civil war, the former sugarcane plantations along the Mississippi River sprouted all manner of communities along The Great River Road. Families in these tight-knit communities enjoyed a seemingly endless supply of local fish, crawfish, and shrimp. Most families were not rich, but many enjoyed productive gardens and good health. The townships are still called parishes just as they were when the regions were first colonized by Roman Catholics from Spain and France. The parishes are named after the Biblical saints, e.g., Gabriel, Charles, John the Baptist, and James. The largest parts of these parishes are now home to over 200 chemical companies and refineries. The fish, crawfish, and shrimp are all gone, and little if anything grows in the poisoned soil and polluted air. Each year, chemical industrial development further encroaches on the majority-Black communities located near the petrochemical complexes. Rates of cancer are documented to be higher than the national average, especially in the African-American communities. Meanwhile, there is virtually no benefit to those communities; jobs in the petrochemical plants are held disproportionately by white residents. Barbara Allen, a professor of science, technology, and society at Virginia Tech puts it this way, “One oppressive economy begets another. The Great River Road was built on the bodies of enslaved Black people. The chemical corridor is responsible for the body burden of their descendants.”
The Heroes of Cancer Alley
Robert Bobby Taylor is a life-time resident of the St. John the Baptist Parish. He told Rebecca Hersher from NPR that he is flabbergasted to be alive today after living 77 years within the borders of the deadly corridor, “‘My mother succumbed to bone cancer. My brother had lung cancer,’ he ticks them off on his fingers. ‘My sister, I think it was cervical cancer. My nephew lung cancer. A favorite cousin. That cousin’s son. Both neighbors on one side, one neighbor on the other. And here I am. I don’t understand how it decides who to take,’” The St. John the Baptist Parish lies adjacent to the Denka neoprene plant (formerly owned by Dupont), which manufactures chloroprene, classified as a “suspected human carcinogen” by the Environmental Protection Agency (EPA). The Denka plant also emits ethylene oxide, a known human carcinogen. The plant is within 1,500 feet of an elementary school. Those who live within a mile of the plant have the highest cancer risk in the country — more than 700 times the national average in one part of St. John the Baptist Parish, according to EPA estimates. Instead of moving away from his home town, or simply accepting his fate, Bobby Taylor founded a community organization, Concerned Citizens of St. John the Baptist Parish. They are well known for their bright red t-shirts that spell out “Only 0.2 will Do,” referring to the EPA safety limit (in micrograms per cubic meter) for long-term exposure to chloroprene. Concerned Citizens of St. John the Baptist Parish picketed the Denko plant, and Taylor himself became the lead plaintiff in a lawsuit seeking to reduce pollution by Denka. EPA monitoring found levels of chloropene far above the safety limit all over St. John the Baptist Parish. Nevertheless, the lawsuit was thrown out by a federal judge citing failure to file by the deadline. The community continues to gain attention and allies. Most recently, the EPA Administrator, Michael Regan, under the Biden Administration, announced the agency will spend $600,000 to buy “mobile air pollution monitoring equipment” to deploy mainly near St. John the Baptist Parish to measure pollution accurately. St. John the Baptist Parish will need continued help to raise awareness.
To help the communities in St. John the Baptist Parish go to this link, but please read on to better understand our role in contributing to the problem, other victories and heroes, and more ways you can help.
Taylor is not the only one whose family suffered. Up the river, Sharon Lavigne of St. James’ Parish got so sick of burying her friends, neighbors, and family members that she founded the faith-based organization, Rise St. James. Please go to the link to help this tireless group fighting relentlessly for justice for parish residents. As you will read below, they are teaming up with other organizations to prevent yet another petrochemical company, Formosa Plastics, from moving into St. James Parish.
Why Plastics?
In case you wonder why industrial leaders including Russia, are investing in plastic, here is the story in a nutshell: The ingredients that make up plastic come from oil and gas. Plastic manufacturers turn by-products of oil and gas into millions of tons of tiny pellets, called nurdles, and plastic powder, a raw form of vinyl. These are shipped all over the world to make everything from baby bottles to yogurt containers. With the inevitable shift to renewable energy for heating and transportation, the fossil fuel industry will need a different market for selling fossil fuels. Their plan is the creation of a fake market for petroleum-based plastics. In fact, the plastics are already being made, and production is ramping up. Whether we need or or want it, the fossil fuel industry plans to increase plastic production to the point where 35% of all fossil fuel used will be in service of making plastic by the year 2025. You can learn more and help at this link.
Formosa
As a part of this goal to expand the plastic industry (since burning fossil fuels for homes and cars is no longer tenable), Formosa Plastics proposed constructing a new 2,300 acre petrochemical complex in St. James Parish, in additional to their original complex in Baton Rouge. Formosa’s proposed complex would cost 9.4 billion dollars to complete and would include two ethane crackers as well as fourteen attached petrochemical facilities. Formosa is based in Taiwan, and their massive new complex is slated to be built in Northwestern St. James Parish across from already existing facilities. It would be squeezed into the borders of Cancer Alley with absolutely no regard for the health of the residents of the St.James Parish.
The Definition of Environmental Racism
How can they do that? The answer to that questions illustrates the concept of environmental racism, i.e., a form of systemic racism defined as the disproportionate exposure that Indigenous, Black and other racialized communities have to environmental hazards as a result of institutional policies and practices. Formosa did it with help from the local planning commission and parish council. The land owned by Formosa in St. James Parish was re-zoned “residential/industrial” in 2014 by the planning commission and parish council. According to Anne Rolfes of the Louisiana Bucket Brigade, the re-zoning “concentrates all industry in the two highest majority Black districts in the parish.” This fits the definition of environmental racism perfectly. There is no doubt that a new Formosa facility would be devastating, since their existing facility generates waste exceeding more than 800 tons of toxic liquid chemicals, almost 6,500 tons of volatile pollutants that have been linked to respiratory illness, 95 tons of carcinogenic compounds, and over 13.6 million tons of greenhouse gas emission per year. These emission statistics make Formosa one of the largest pollution-causing plants in the entire world. Currently, there is a Formosa plant operating in Baton Rouge from which the data are clear: the effects of having two facilities would more than double the problem. Air pollution would skyrocket and the pollutants would include nitrogen oxides, carbon monoxide, ozone, sulfur dioxide, and particulate matter. Nitrogen oxides can lead to new or worsening respiratory conditions and exaggerate pre-existing cardiac conditions. Carbon monoxide can aggravate cardiac symptoms and conditions, potentially leading to premature death. Ozone, or as it is often referred to, smog, can lead to new or worsening respiratory illness. Sulfur dioxide can lead to respiratory conditions and other ailments. Particulate matter can cause premature death in individuals with pre-existing respiratory and cardiac conditions.
In addition to these pollutants leading to ailments within communities, if Formosa Plastics Corporations were to expand into St.James, the residents would be more likely to develop multiple kinds of cancer. According to the Stop Formosa Organization, air pollution from this plastics plant would include ethylene oxide (a carcinogen that is known to cause breast cancer, non-Hodgkin lymphoma, and lymphocytic leukemia), benzene (carcinogen), vinyl chloride (hepatic angiosarcoma, leukemia, lymphoma, brain, and lung cancers), formaldehyde is (myeloid leukemia and other rare cancers), acetaldehyde (probable carcinogen). All of these pollutants are causing substantial harm to the environment (plants and animals are disappearing), and the people who live there are experiencing illness and premature death.
Considering these emissions, it is not surprising that the COVID-19 pandemic has hit Cancer Alley harder than most areas. Since these pollutants often lead to respiratory weakness and disease, higher COVID death rates follow. Areas such as St. John, Louisiana, which has the highest concentration of particulate matter, as well as the highest rate of COVID deaths, is an example of how these pollutants not only cause harm on their own but also interact with other diseases.
Victories
The struggle of Sharon Lavigne and Rise St. James was no picnic. Formosa plastics denied that any illness or death in St. James Parish was directly related to plastics production (they conducted their own studies to bolster their defense), and the company used a number of strategies to silence the St. James community. Lavigne says that many of her neighbors were afraid to speak up, and some who lacked an alternative means of livelihood were afraid to lose their jobs with Formosa. During the COVID pandemic, it was difficult to meet and carry out their plans. The odds continued to stack up against Rise St. James when the state legislature passed a bill requiring judges to impose a mandatory three-year minimum prison sentence with hard labor on protesters convicted of trespassing on industrial property or infrastructure. “Industrial property” was defined as “anywhere in a state with 125,000 miles of oil and gas pipelines,” which basically includes all of St. James Parish. It is beyond inspiring that Lavigne and Rise St. James persisted in the face of these obstacles.
Eventually, however, the bill to squelch St. James protests was vetoed by the governor. On April 8, 2021, the New Orleans City Council voted unanimously to declare its opposition. Unlike the city council, the Biden administration’s commitments are more than symbolic, and the Formosa project has been forestalled, at least for now. The victories were thanks to Sharon Lavigne, members of the St. James community, hard-working organizations like Rise St. James, the Biden administration, and the United Nations. The United States Army Corps of Engineers is the agency that grants construction permits per the Clean Water Act and the primary federal enforcers of regulations regarding water pollution. In August 2021, it announced that they would commission a full environmental impact statement, and this process will put a halt to future construction of the Formosa Plastics complex in St. James Parish for a number of years. Most recently, Formosa has been put on notice by Standard & Poor’s that the delays have actually helped its credit rating compared to its credit rating when the project was first announced. This implies that cancelling the project would be more profitable for the company than building the plant in St. James Parish. This is good news for St. James Parish, and vividly illustrates that industrial leaders pay attention to profits, not people. Sharon Lavigne shows us how to move forward even against all odds. Her strategy is one of perspective; she sees this development as a period of momentum in the trajectory of the environmental justice movement. Lavigne says, “Nobody took it upon themselves to speak for St James Parish until we started working to stop Formosa Plastics. Now the world is watching this important victory for environmental justice.”
WAYS YOU CAN HELP: Plastic Pollution Coalition